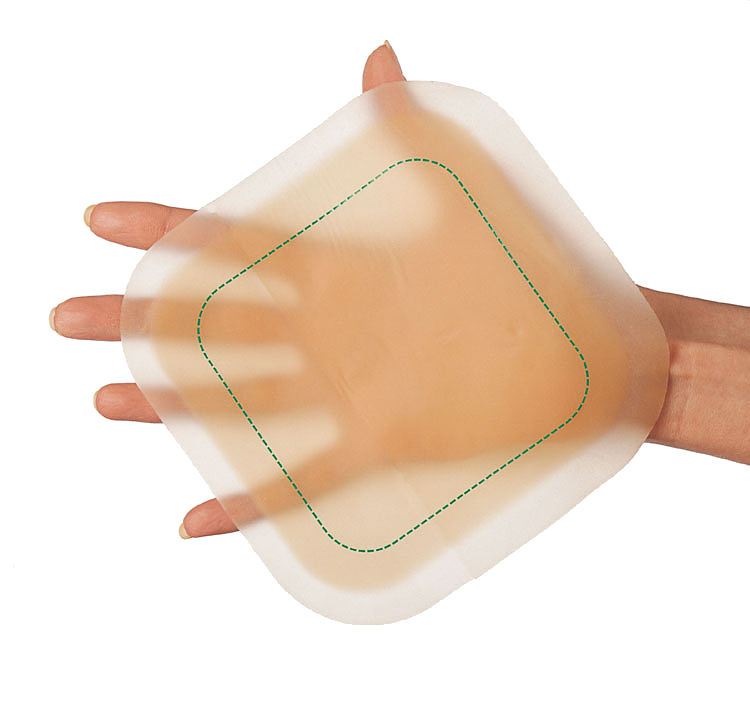
Consists of a layer of hydrocolloid laminated to a breathable film, and a polyurethane foam backing. Does not contain animal-derived ingredients.
Lightly to moderately exuding chronic and acute wounds, including pressure ulcers, venous leg ulcers, diabetic foot ulcers, lacerations, abrasions and postoperative surgical wounds. Can be used under compression bandages and in cavity wounds as a secondary dressing.
Individuals with a known sensitivity to hydrocolloids

Consists of a thin layer of hydrocolloid laminated to a highly breathable film. Contains a mixture of synthetic polymers and hydrophilic powders, with a high moisture vapour transmission rate film backing. Does not contain animal-derived ingredients.
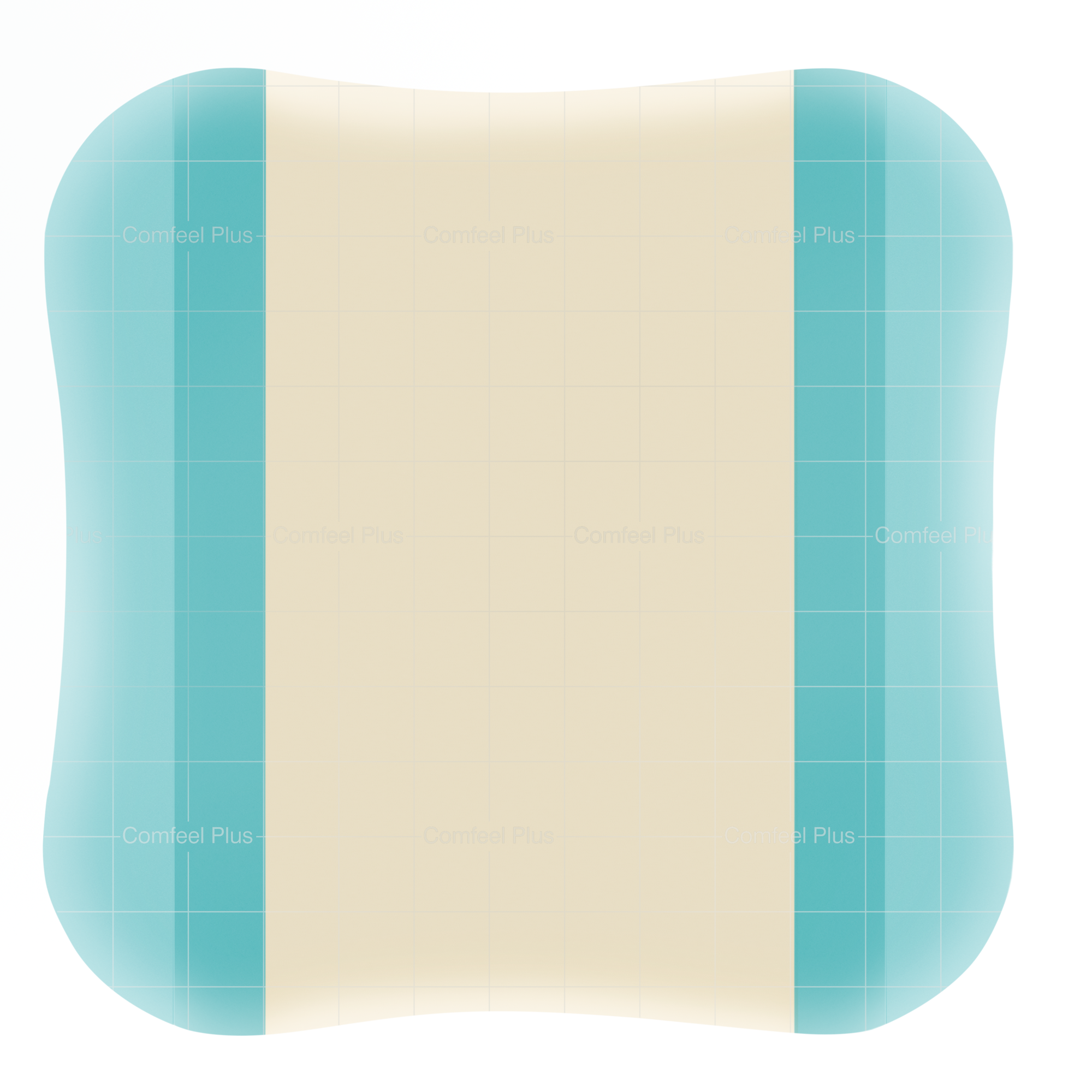
Absorbent hydrocolloid dressing with added alginate for absorption, a vapour-permeable film backing and bevelled edge to reduce the risk of rolling edges. Mapping grid to aid wound measurement.
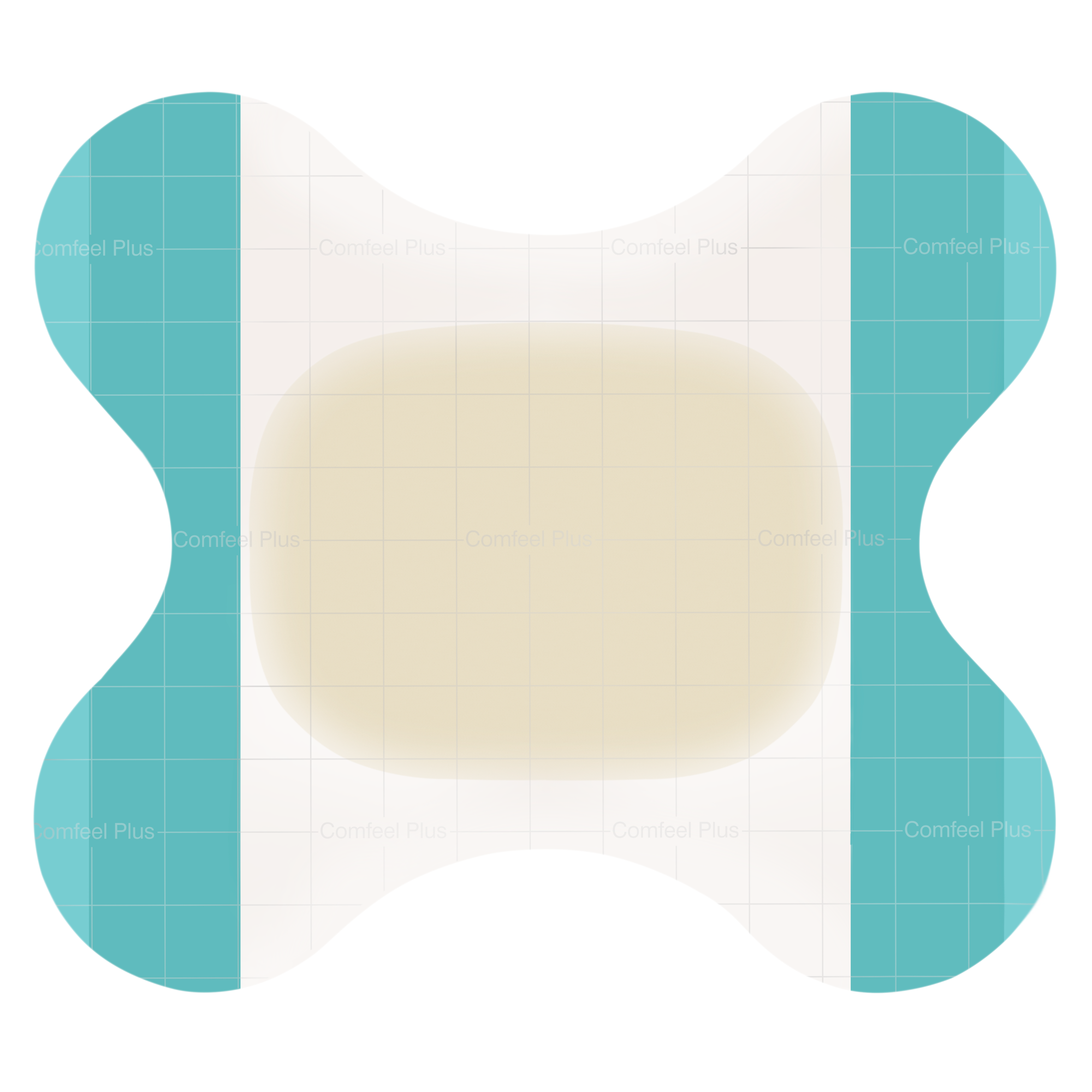
Absorbent hydrocolloid dressing with added alginate for absorption, a vapour-permeable film backing and a bevelled edge to reduce the risk of rolling edges. Mapping grid to aid wound measurement.
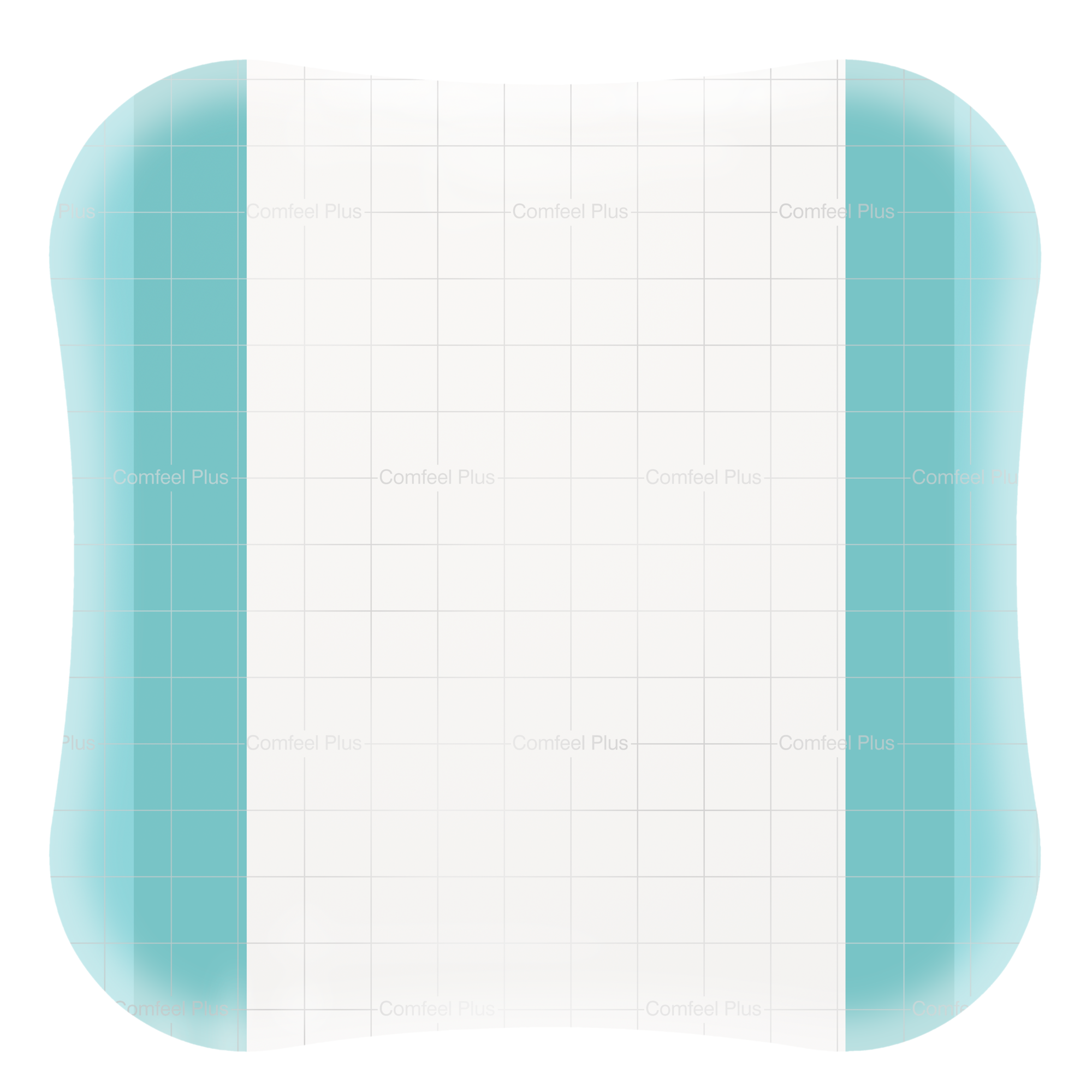
Absorbent, transparent hydrocolloid dressing with a vapour-permeable film backing and bevelled edge to reduce the risk of rolling edges. Consists of moisture absorbing sodium carboxymethylcellulose (CMC) particles encapsulated in a synthetic, elastic and sticky mass. The top film is a semi-permeable polyurethane film. Mapping grid to aid wound measurement.