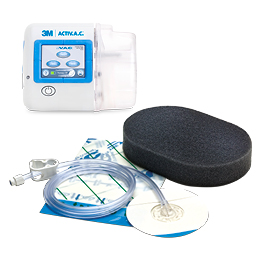
When used with VACUlta, provides an active temporary abdominal closure system that is designed to remove fluids from the abdominal cavity and draw wound edges together, helping to achieve primary fascial closure while protecting abdominal contents from external contaminates
Temporary bridging of abdominal wall openings where primary closure is not possible and/or repeat abdominal entries are necessary. Open abdominal wounds with exposed viscera including, but not limited to, abdominal compartment syndrome. Should be used in a closely monitored area within the acute care hospital, such as the ICU. Is most often be applied in the operating theatre.
Never place exposed foam material in direct contact with exposed bowel, organs, blood vessels or nerves. Protect vital structures with the visceral protective layer at all times during therapy. Do not use on patients with open abdominal wounds containing non-enteric unexplored fistulas.